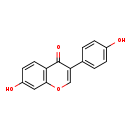
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-001j-1928000000-f956e5fd0e616ef96161 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-000t-1619000000-eb2f87cd7d535b4d0446 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-001j-1928000000-f956e5fd0e616ef96161 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-000t-1619000000-eb2f87cd7d535b4d0446 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-001j-1927000000-87f11afa0cde5b353147 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-1390000000-0ff8f0de4d2d339f9357 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00gi-4229000000-71c73470904ad7b680ee | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0udi-0090000000-20d2c8ade6aac1f46e8a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0udi-0970000000-a2349152106bd9da1257 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0006-9100000000-691c394958fd19d47510 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-APPI-QQ (API2000) , Positive | splash10-0f7x-4900000000-5acb56232432597c00e5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-APPI-QQ (API2000) , Positive | splash10-0pej-1930000000-89bedfb39603a12f3c75 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-APPI-QQ (API2000) , Positive | splash10-0a4i-0490000000-c633d3449d8bb0206fc2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-APPI-QQ (API2000) , Positive | splash10-0a4i-0190000000-3a5de741e814e47d5cbe | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-APPI-QQ (API2000) , Positive | splash10-0a4i-0090000000-fb84819005f7f65b4fc8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-APPI-QQ (API2000) , Positive | splash10-0a4i-0090000000-b3ae695d217c756a5f8f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 5V, Positive | splash10-0a4i-0090000000-d25bba2f5a1ac12e3d86 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-0udi-0190000000-a9b6c8c416cb482a5dc1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-0089-1970000000-f6070acf0fd663c4ae6f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0uem-1900000000-fa199caebb806e9eb0f9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0a4i-0490000000-3defe1cbaf29e0cd2d1f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0a4i-1790000000-2502633932fc66220039 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-0udi-0290000000-56b4c039a64ab57d3e70 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Negative | splash10-0089-2970000000-f32e85ba94cb6621d4f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-7dcbed418d38de120895 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0090000000-c522d590074eabe8c4c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002r-6950000000-7b85969731514bf2d0bc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-388d21c85a84d4e0988a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-cc8371583b5ba50a4f0d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gbi-3950000000-cb120c48a579b9363f74 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-7dcbed418d38de120895 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0090000000-c522d590074eabe8c4c4 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|