| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:03:21 UTC |
|---|
| Update Date | 2020-05-11 20:53:47 UTC |
|---|
| BMDB ID | BMDB0003254 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Muramic acid |
|---|
| Description | Muramic acid, also known as muramate, belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. Based on a literature review a significant number of articles have been published on Muramic acid. |
|---|
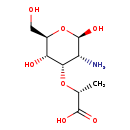
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Muramate | Generator | | (+)-Muramate | HMDB | | (+)-Muramic acid | HMDB | | 3-O-alpha-Carboxyethyl-D-glucosamine | HMDB | | 3-O-alpha-Carboxyethyl-delta-glucosamine | HMDB | | Muramic acid hydrate | HMDB | | Murexide | HMDB | | (2R)-2-{[(2R,3R,4S,5S,6R)-3-amino-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}propanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C9H17NO7 |
|---|
| Average Molecular Weight | 251.2338 |
|---|
| Monoisotopic Molecular Weight | 251.100501903 |
|---|
| IUPAC Name | (2R)-2-{[(2R,3R,4S,5S,6R)-3-amino-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}propanoic acid |
|---|
| Traditional Name | (2R)-2-{[(2R,3R,4S,5S,6R)-3-amino-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}propanoic acid |
|---|
| CAS Registry Number | 1114-41-6 |
|---|
| SMILES | C[C@@H](O[C@H]1[C@@H](N)[C@H](O)O[C@H](CO)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H17NO7/c1-3(8(13)14)16-7-5(10)9(15)17-4(2-11)6(7)12/h3-7,9,11-12,15H,2,10H2,1H3,(H,13,14)/t3-,4-,5-,6-,7+,9-/m1/s1 |
|---|
| InChI Key | MSFSPUZXLOGKHJ-LEISLEKSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Muramic_acid
- Hexose monosaccharide
- Amino saccharide
- Sugar acid
- Monosaccharide
- Oxane
- Amino acid or derivatives
- Hemiacetal
- Amino acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Primary aliphatic amine
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Carbonyl group
- Hydrocarbon derivative
- Organonitrogen compound
- Alcohol
- Primary alcohol
- Primary amine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 153 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-003r-9520000000-91bf5f5e77865f5b146f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-00fr-5722490000-e41bf810e5d7a6b7be4b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0190000000-d990980701f630613360 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-5950000000-a7012f82e4f7a8356db2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01xx-9500000000-9b72e362c6da0556143a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udr-7390000000-24dfc3347bddf790ce96 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-9830000000-eac28b7b8efa7e1d642a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007d-9400000000-8a1ef61dfc5b55d91d45 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-8ffd847778e88673df79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-007c-9000000000-bb66080cf35469c7116f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-9000000000-dec404b78b57490bbf3a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0190000000-2826fd1498ec2b1ac21d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0il0-2940000000-9cd55fcc2e559c11de76 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fxx-9800000000-6d882558ee7ae32f13f8 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|