| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:03:00 UTC |
|---|
| Update Date | 2020-04-22 15:11:47 UTC |
|---|
| BMDB ID | BMDB0003166 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 16b-Hydroxystanozolol |
|---|
| Description | 16b-Hydroxystanozolol belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. Based on a literature review a small amount of articles have been published on 16b-Hydroxystanozolol. |
|---|
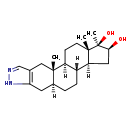
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 16-Hydroxystanozolol | MeSH | | 16-Hydroxystanozolol, (5alpha,16alpha,17alpha)-isomer | MeSH | | 16-Hydroxystanozolol, (5alpha,16alpha,17beta)-isomer | MeSH | | (5a,16b,17b)-17-Methyl-'h-androst-2-eno[3,2-c]pyrazole-16,17-diol | HMDB | | 16b-OH-Stanozolol | HMDB | | 16beta-Hydroxystanozolol | HMDB | | Cyclopenta[7,8]phenanthro[2,3-c]pyrazole, 2'H-androst-2-eno[3,2-c]pyrazole-16,17-diol deriv. | HMDB |
|
|---|
| Chemical Formula | C21H32N2O2 |
|---|
| Average Molecular Weight | 344.491 |
|---|
| Monoisotopic Molecular Weight | 344.246378278 |
|---|
| IUPAC Name | (1S,2S,10S,13R,14S,16S,17R,18S)-2,17,18-trimethyl-6,7-diazapentacyclo[11.7.0.0²,¹⁰.0⁴,⁸.0¹⁴,¹⁸]icosa-4(8),5-diene-16,17-diol |
|---|
| Traditional Name | (1S,2S,10S,13R,14S,16S,17R,18S)-2,17,18-trimethyl-6,7-diazapentacyclo[11.7.0.0²,¹⁰.0⁴,⁸.0¹⁴,¹⁸]icosa-4(8),5-diene-16,17-diol |
|---|
| CAS Registry Number | 125590-76-3 |
|---|
| SMILES | [H][C@@]12C[C@H](O)[C@](C)(O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC3=C(C[C@]12C)C=NN3 |
|---|
| InChI Identifier | InChI=1S/C21H32N2O2/c1-19-10-12-11-22-23-17(12)8-13(19)4-5-14-15(19)6-7-20(2)16(14)9-18(24)21(20,3)25/h11,13-16,18,24-25H,4-10H2,1-3H3,(H,22,23)/t13-,14+,15-,16-,18-,19-,20-,21-/m0/s1 |
|---|
| InChI Key | IZGBPAAEPVNBGA-BWPSUJIGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrane steroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrane-skeleton
- Hydroxysteroid
- 16-beta-hydroxysteroid
- 16-hydroxysteroid
- 17-hydroxysteroid
- Azole
- Cyclic alcohol
- Pyrazole
- Tertiary alcohol
- Heteroaromatic compound
- 1,2-diol
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 2.801 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uxs-1298000000-e5922c5f2c2da69632b5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-3236900000-157d8d09efaeaf84d26b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-3dce83afdfd62c3562f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ar0-0093000000-d8197d56bb13cd018ce9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ldi-7987000000-c63f25f5fb379062b178 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-d62368294f0bd1560d22 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mo-0009000000-1b482a36251fc401712a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-2049000000-6cd31c36847fe353db02 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0029000000-eecedc58c6f30dab7690 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000j-0964000000-f58bdd1b840402e74de4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0590000000-c98f4d460e53eb3a27b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-f0c9f6f6d6de10398e83 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0049000000-0ebdb8c09f289f5ce0bb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0029000000-60ec668969053e23118d | View in MoNA |
|---|
|
|---|