| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:02:41 UTC |
|---|
| Update Date | 2020-05-05 18:40:24 UTC |
|---|
| BMDB ID | BMDB0003070 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Shikimic acid |
|---|
| Description | Shikimic acid, also known as shikimate or acid, shikimic, belongs to the class of organic compounds known as shikimic acids and derivatves. These are cyclitols containing a cyclohexanecarboxylic acid substituted with three hydroxyl groups at positions 3, 4, and 5. Shikimic acid exists in all living species, ranging from bacteria to plants to humans. Based on a literature review a significant number of articles have been published on Shikimic acid. |
|---|
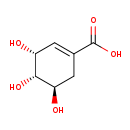
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4,5-Trihydroxy-1-cyclohexenecarboxylic acid | ChEBI | | 3alpha,4alpha,5beta-Trihydroxy-1-cyclohexene-1-carboxylic acid | ChEBI | | [3R-(3alpha,4alpha,5beta)]-3,4,5-Trihydroxy-1-cyclohexene-1-carboxylic acid | ChEBI | | L-Shikimic acid | ChEBI | | Shikimate | ChEBI | | 3,4,5-Trihydroxy-1-cyclohexenecarboxylate | Generator | | 3a,4a,5b-Trihydroxy-1-cyclohexene-1-carboxylate | Generator | | 3a,4a,5b-Trihydroxy-1-cyclohexene-1-carboxylic acid | Generator | | 3alpha,4alpha,5beta-Trihydroxy-1-cyclohexene-1-carboxylate | Generator | | 3Α,4α,5β-trihydroxy-1-cyclohexene-1-carboxylate | Generator | | 3Α,4α,5β-trihydroxy-1-cyclohexene-1-carboxylic acid | Generator | | [3R-(3a,4a,5b)]-3,4,5-Trihydroxy-1-cyclohexene-1-carboxylate | Generator | | [3R-(3a,4a,5b)]-3,4,5-Trihydroxy-1-cyclohexene-1-carboxylic acid | Generator | | [3R-(3alpha,4alpha,5beta)]-3,4,5-Trihydroxy-1-cyclohexene-1-carboxylate | Generator | | [3R-(3Α,4α,5β)]-3,4,5-trihydroxy-1-cyclohexene-1-carboxylate | Generator | | [3R-(3Α,4α,5β)]-3,4,5-trihydroxy-1-cyclohexene-1-carboxylic acid | Generator | | L-Shikimate | Generator | | Acid, shikimic | MeSH | | (-)-Shikimate | HMDB | | (-)-Shikimic acid | HMDB | | Skikimate | HMDB | | Skikimic acid | HMDB | | (3R,4S,5R)-3,4,5-Trihydroxy-1-cyclohexene-1-carboxylic acid | PhytoBank | | (-)-3,4,5-Trihydroxy-1-cyclohexene-1-carboxylic acid | PhytoBank | | Shikimic acid | PhytoBank |
|
|---|
| Chemical Formula | C7H10O5 |
|---|
| Average Molecular Weight | 174.1513 |
|---|
| Monoisotopic Molecular Weight | 174.05282343 |
|---|
| IUPAC Name | (3R,4S,5R)-3,4,5-trihydroxycyclohex-1-ene-1-carboxylic acid |
|---|
| Traditional Name | (-)-shikimate |
|---|
| CAS Registry Number | 138-59-0 |
|---|
| SMILES | O[C@@H]1CC(=C[C@@H](O)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H10O5/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1,4-6,8-10H,2H2,(H,11,12)/t4-,5-,6-/m1/s1 |
|---|
| InChI Key | JXOHGGNKMLTUBP-HSUXUTPPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as shikimic acids and derivatves. These are cyclitols containing a cyclohexanecarboxylic acid substituted with three hydroxyl groups at positions 3, 4, and 5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Shikimic acids and derivatves |
|---|
| Alternative Parents | |
|---|
| Substituents | - Shikimic acid or derivatives
- Secondary alcohol
- Polyol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 186 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 150 mg/mL at 21 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-0udj-0970000000-42465cd3f3e138b0bc12 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0udi-0790000000-1100443abb8605953f58 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-00di-9450000000-e6ca954dc1a9c1cc4285 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-0udi-0491000000-49993b9b18e12b9461fc | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-0391000000-ddb2c574233062a911fa | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0udj-0970000000-42465cd3f3e138b0bc12 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0udi-0790000000-1100443abb8605953f58 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9450000000-e6ca954dc1a9c1cc4285 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0udi-0491000000-49993b9b18e12b9461fc | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-4900000000-817f1ed3feb4c763285f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0092-7119400000-67b0989b5019a72e1016 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-01b9-1900000000-fd80f5e7f51d927e8e15 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0a4i-9300000000-cf8a3148fd132540bf97 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0a4i-9000000000-ed28bc20ae43473043c9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0a4i-9000000000-65249fc24f2de4acf2c7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0a4i-9000000000-26dfc876ae35c2cdb845 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-006x-9600000000-3a5ab91754d9d837d8b4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-01b9-1900000000-6cba5b9b7c4891522045 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0a4i-9300000000-12d15a049b141a37f34e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0a4i-9000000000-ed28bc20ae43473043c9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0a4i-9000000000-9864f7359ffed65d6ef0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0a4i-9000000000-26dfc876ae35c2cdb845 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-006x-9600000000-3a5ab91754d9d837d8b4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-00di-7900000000-d77a830354efe8731a42 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-01vx-9700000000-d2060c544171987e3dcd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-01vx-9500000000-baa64c3938f3d039c6e4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-d5821372fd8a830dc962 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-9000000000-cb13aba83ba37150b931 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-01vo-9600000000-3433190ea61f16d92efd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-022l-9600000000-60e85b9b59edb135e4c4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-58e1775d1e013c416201 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-1f370d2806fd8245ebfb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-01vx-9600000000-d502f575cada8c0b5aea | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-00dl-9400000000-d0ff38d5a290b8e8248d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-004i-0900000000-e6ca4ce5acb44dc23a74 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-004r-9700000000-18afe769b0ef5ba3fbdb | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, D2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 1H]-TOCSY. Unexported temporarily by An Chi on Oct 15, 2021 until json or nmrML file is generated. 2D NMR Spectrum (experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|