| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:02:33 UTC |
|---|
| Update Date | 2020-05-11 19:57:21 UTC |
|---|
| BMDB ID | BMDB0003039 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Isoacitretin |
|---|
| Description | Isoacitretin, also known as 13-cis-acitretin or soriatane, belongs to the class of organic compounds known as retinoids. These are oxygenated derivatives of 3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)nona-1,3,5,7-tetraene and derivatives thereof. Based on a literature review a significant number of articles have been published on Isoacitretin. |
|---|
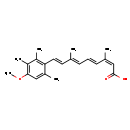
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 13-cis-Acitretin | HMDB | | 13-cis-Etretin | HMDB | | Soriatane | HMDB | | Acitretin | HMDB | | Acitretin andreu brand | HMDB | | Acitretin roche brand | HMDB | | Acitretin, (Z,e,e,e)-isomer | HMDB | | Andreu brand OF acitretin | HMDB | | Etretin | HMDB | | Hoffmann la roche brand OF acitretin | HMDB | | Hoffmann-la roche brand OF acitretin | HMDB | | Isoetretin | HMDB | | Neotigason | HMDB | | Roche brand OF acitretin | HMDB | | (2Z,4E,6E,8E)-9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid | HMDB | | Isoacitretin | MeSH |
|
|---|
| Chemical Formula | C21H26O3 |
|---|
| Average Molecular Weight | 326.4293 |
|---|
| Monoisotopic Molecular Weight | 326.188194698 |
|---|
| IUPAC Name | (2Z,4E,6E,8E)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid |
|---|
| Traditional Name | acetretin |
|---|
| CAS Registry Number | 69427-46-9 |
|---|
| SMILES | COC1=C(C)C(C)=C(\C=C\C(\C)=C\C=C\C(\C)=C/C(O)=O)C(C)=C1 |
|---|
| InChI Identifier | InChI=1S/C21H26O3/c1-14(8-7-9-15(2)12-21(22)23)10-11-19-16(3)13-20(24-6)18(5)17(19)4/h7-13H,1-6H3,(H,22,23)/b9-7+,11-10+,14-8+,15-12- |
|---|
| InChI Key | IHUNBGSDBOWDMA-UGOGCBOOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as retinoids. These are oxygenated derivatives of 3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)nona-1,3,5,7-tetraene and derivatives thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Retinoids |
|---|
| Direct Parent | Retinoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Retinoic acid
- Retinoid skeleton
- Sesquiterpenoid
- Cyclofarsesane sesquiterpenoid
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Medium-chain fatty acid
- Styrene
- Alkyl aryl ether
- Branched fatty acid
- Methyl-branched fatty acid
- Monocyclic benzene moiety
- Fatty acyl
- Fatty acid
- Benzenoid
- Unsaturated fatty acid
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Carbonyl group
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-1279000000-d74bbc0348cfdb3b3732 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-001i-4139000000-85e857f30a345651bb66 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-01t9-0592000000-92a48c620c961fdb430f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-056r-1943000000-77c18bcac662a9f6bdcb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-056r-2942000000-57cdf186555912258599 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0369000000-c76928c945feff099218 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-0970000000-83c7baa1aeda7da5a2cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pvi-4910000000-b657ca2b064b9ff56256 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0059-0049000000-4a4ee208619c94b4e2c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-057i-0079000000-35044b92edd7238b81e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-066u-2291000000-5bb79aadc2928c46ff88 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001j-0092000000-da6b1640ed88e8dd0629 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-017j-1291000000-8d03b8e6cfa817060924 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dj-1494000000-45aa9916567486569a90 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ke9-0395000000-2d28d3c86a8a16a5adac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0940000000-2f4d9396336fd697861e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00tf-1900000000-f5a87d3a7b17af3487e6 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Mestres Quadreny, Ramon; Tortajada Lopez, Desamparados; Arrell Piquer, Maria Jose; Parra Alvarez, Margarita; Gil Grau, Salvador; Cetta Builelo, Luisa; Simo Planells, Ana. Process for the preparation of aromatic retinoic acids [e.g., etretin] and their derivatives. Span. (1992), 14 pp. |
|---|