| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:00:53 UTC |
|---|
| Update Date | 2020-04-22 15:11:10 UTC |
|---|
| BMDB ID | BMDB0002580 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Taurolithocholic acid 3-sulfate |
|---|
| Description | Taurolithocholic acid 3-sulfate, also known as 3alpha-sulfatolithocholyltaurine or SLCT-3-sulfate, belongs to the class of organic compounds known as taurinated bile acids and derivatives. These are bile acid derivatives containing a taurine conjugated to the bile acid moiety. Based on a literature review a significant number of articles have been published on Taurolithocholic acid 3-sulfate. |
|---|
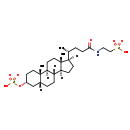
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3alpha-Sulfato-5beta-cholan-24-oyl)-2'-aminoethanesulfonate | ChEBI | | 3alpha-Sulfatolithocholyltaurine | ChEBI | | SLCT-3-Sulfate | ChEBI | | Taurolithocholate 3-sulfate | ChEBI | | Taurolithocholate sulfate | ChEBI | | TLC-S | ChEBI | | (3a-Sulfato-5b-cholan-24-oyl)-2'-aminoethanesulfonate | Generator | | (3a-Sulfato-5b-cholan-24-oyl)-2'-aminoethanesulfonic acid | Generator | | (3a-Sulphato-5b-cholan-24-oyl)-2'-aminoethanesulphonate | Generator | | (3a-Sulphato-5b-cholan-24-oyl)-2'-aminoethanesulphonic acid | Generator | | (3alpha-Sulfato-5beta-cholan-24-oyl)-2'-aminoethanesulfonic acid | Generator | | (3alpha-Sulphato-5beta-cholan-24-oyl)-2'-aminoethanesulphonate | Generator | | (3alpha-Sulphato-5beta-cholan-24-oyl)-2'-aminoethanesulphonic acid | Generator | | (3Α-sulfato-5β-cholan-24-oyl)-2'-aminoethanesulfonate | Generator | | (3Α-sulfato-5β-cholan-24-oyl)-2'-aminoethanesulfonic acid | Generator | | (3Α-sulphato-5β-cholan-24-oyl)-2'-aminoethanesulphonate | Generator | | (3Α-sulphato-5β-cholan-24-oyl)-2'-aminoethanesulphonic acid | Generator | | 3a-Sulfatolithocholyltaurine | Generator | | 3a-Sulphatolithocholyltaurine | Generator | | 3alpha-Sulphatolithocholyltaurine | Generator | | 3Α-sulfatolithocholyltaurine | Generator | | 3Α-sulphatolithocholyltaurine | Generator | | SLCT-3-Sulfuric acid | Generator | | SLCT-3-Sulphate | Generator | | SLCT-3-Sulphuric acid | Generator | | Taurolithocholate 3-sulphate | Generator | | Taurolithocholic acid 3-sulfuric acid | Generator | | Taurolithocholic acid 3-sulphuric acid | Generator | | Taurolithocholate sulphate | Generator | | Taurolithocholic acid sulfuric acid | Generator | | Taurolithocholic acid sulphuric acid | Generator | | Taurolithocholic acid 3-sulphate | HMDB | | Taurolithocholic acid sulfate | HMDB | | Taurolithocholic acid sulphate | HMDB | | Taurolithocholic acid 3-sulfate | ChEBI |
|
|---|
| Chemical Formula | C26H45NO8S2 |
|---|
| Average Molecular Weight | 563.767 |
|---|
| Monoisotopic Molecular Weight | 563.258658801 |
|---|
| IUPAC Name | 2-[(4R)-4-[(1S,2S,5R,7R,10R,11S,14R,15R)-2,15-dimethyl-5-(sulfooxy)tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]ethane-1-sulfonic acid |
|---|
| Traditional Name | 2-[(4R)-4-[(1S,2S,5R,7R,10R,11S,14R,15R)-2,15-dimethyl-5-(sulfooxy)tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]ethanesulfonic acid |
|---|
| CAS Registry Number | 15324-65-9 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OS(O)(=O)=O)[C@H](C)CCC(=O)NCCS(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C26H45NO8S2/c1-17(4-9-24(28)27-14-15-36(29,30)31)21-7-8-22-20-6-5-18-16-19(35-37(32,33)34)10-12-25(18,2)23(20)11-13-26(21,22)3/h17-23H,4-16H2,1-3H3,(H,27,28)(H,29,30,31)(H,32,33,34)/t17-,18-,19-,20+,21-,22+,23+,25+,26-/m1/s1 |
|---|
| InChI Key | HSNPMXROZIQAQD-GBURMNQMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as taurinated bile acids and derivatives. These are bile acid derivatives containing a taurine conjugated to the bile acid moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Taurinated bile acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Taurinated bile acid
- Sulfated steroid skeleton
- Fatty amide
- N-acyl-amine
- Sulfuric acid monoester
- Sulfate-ester
- Fatty acyl
- Sulfuric acid ester
- Alkyl sulfate
- Organic sulfuric acid or derivatives
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Sulfonyl
- Alkanesulfonic acid
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid derivative
- Carbonyl group
- Organopnictogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001s-0122960000-7322e2c8b4da02bf9e0a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-001i-0000900000-4d1082945d64455a6eab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900340000-82769bc714a44b90e5d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1900200000-44fc18bc9bafc7c398f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-7911100000-5bfee268d4d671bbc025 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-3100490000-7c2e69684854500bbe5f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q9-5201920000-f65c49dc30c74c2b1eb9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0089-9303100000-f4bac240298f8ba5004f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000590000-1b81180dda2f5166dc7f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0219500000-ec90bdc61d5b63c6bb94 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p9-4279200000-8776b4d5f63c61813c12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000090000-baf7d898c278a1ba0a12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-9000050000-c8bf230c02d78676120c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01qa-9000030000-78391f8c8560da71030e | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|