| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:00:46 UTC |
|---|
| Update Date | 2020-04-22 15:11:08 UTC |
|---|
| BMDB ID | BMDB0002536 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Isodeoxycholic acid |
|---|
| Description | Isodeoxycholic acid, also known as isodeoxycholate, belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. Based on a literature review a significant number of articles have been published on Isodeoxycholic acid. |
|---|
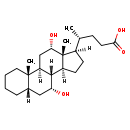
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7alpha,12alpha-Dihydroxy-5beta-cholanic acid | Kegg | | 7alpha,12alpha-Dihydroxycholanoic acid | Kegg | | 7a,12a-Dihydroxy-5b-cholanate | Generator | | 7a,12a-Dihydroxy-5b-cholanic acid | Generator | | 7alpha,12alpha-Dihydroxy-5beta-cholanate | Generator | | 7Α,12α-dihydroxy-5β-cholanate | Generator | | 7Α,12α-dihydroxy-5β-cholanic acid | Generator | | 7a,12a-Dihydroxycholanoate | Generator | | 7a,12a-Dihydroxycholanoic acid | Generator | | 7alpha,12alpha-Dihydroxycholanoate | Generator | | 7Α,12α-dihydroxycholanoate | Generator | | 7Α,12α-dihydroxycholanoic acid | Generator | | Isodeoxycholate | Generator | | 3-Deoxycholic acid | HMDB | | 7a,12a-Dihydroxy-5b-cholan-24-Oate | HMDB | | 7a,12a-Dihydroxy-5b-cholan-24-Oic acid | HMDB | | 7a,12a-Dihydroxy-5b-cholanoate | HMDB | | 7a,12a-Dihydroxy-5b-cholanoic acid | HMDB | | 7alpha,12alpha-Dihydroxy-5beta-cholan-24-Oate | HMDB | | 7alpha,12alpha-Dihydroxy-5beta-cholan-24-Oic acid | HMDB | | Isodeoxycholic acid | KEGG | | (4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-9,16-Dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C24H40O4 |
|---|
| Average Molecular Weight | 392.572 |
|---|
| Monoisotopic Molecular Weight | 392.292659768 |
|---|
| IUPAC Name | (4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-9,16-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoic acid |
|---|
| Traditional Name | (4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R,16S)-9,16-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoic acid |
|---|
| CAS Registry Number | 566-17-6 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])CCCC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C24H40O4/c1-14(7-10-21(27)28)16-8-9-17-22-18(13-20(26)24(16,17)3)23(2)11-5-4-6-15(23)12-19(22)25/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17+,18+,19-,20+,22+,23+,24-/m1/s1 |
|---|
| InChI Key | ZHCAAZIHTDCFJX-QLEQUTGBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydroxy bile acid, alcohol, or derivatives
- 12-hydroxysteroid
- Hydroxysteroid
- 7-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01r2-1349000000-c20944bdb515b39b1d8b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0006-1110190000-4fbd3514c97db8f97c4d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0009000000-875537fa9339c3a3bbec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-1009000000-20a7c31d6177cdac7ecc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02vj-7009000000-e32583ec8a96a2d6796d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-a7e668c25bb4e322f429 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-1009000000-af1365631dc58c1b7979 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9006000000-33d068d8853add57effa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-e572c29938091d5ba610 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05bf-5297000000-e9bd316f7bfb3538706a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-9530000000-343097ad815cfbd487e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-7eba834115ee2c679208 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-0009000000-03834bc03b5a1c28e5f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1039000000-b5eba12dd80348c13a4d | View in MoNA |
|---|
|
|---|