| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:55:05 UTC |
|---|
| Update Date | 2020-04-22 15:10:56 UTC |
|---|
| BMDB ID | BMDB0002395 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
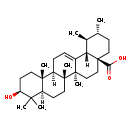
| Common Name | Ursolic acid |
|---|
| Description | Ursolic acid, also known as malol or prunol, belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. Based on a literature review a significant number of articles have been published on Ursolic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3beta)-3-Hydroxyurs-12-en-28-Oic acid | ChEBI | | Malol | ChEBI | | Prunol | ChEBI | | Urson | ChEBI | | (3b)-3-Hydroxyurs-12-en-28-Oate | Generator | | (3b)-3-Hydroxyurs-12-en-28-Oic acid | Generator | | (3beta)-3-Hydroxyurs-12-en-28-Oate | Generator | | (3Β)-3-hydroxyurs-12-en-28-Oate | Generator | | (3Β)-3-hydroxyurs-12-en-28-Oic acid | Generator | | Ursolate | Generator | | (3beta)-3-Hydroxy-urs-12-en-28-Oate | HMDB | | (3beta)-3-Hydroxy-urs-12-en-28-Oic acid | HMDB | | 3beta-Hydroxy-12-ursen-28-ic acid | HMDB | | 3beta-Hydroxy-urs-12-en-28-Oate | HMDB | | 3beta-Hydroxy-urs-12-en-28-Oic acid | HMDB | | 3beta-Hydroxyurs-12-en-28-Oate | HMDB | | 3beta-Hydroxyurs-12-en-28-Oic acid | HMDB | | 3-Epi-ursolic acid | HMDB | | Merotaine | HMDB | | (+)-Ursolic acid | HMDB | | 3Β-hydroxyurs-12-en-28-Oic acid | HMDB | | beta-Ursolic acid | HMDB | | Β-ursolic acid | HMDB | | Bungeolic acid | HMDB | | Ursolisome | HMDB | | UA | HMDB | | Ursolic acid | HMDB |
|
|---|
| Chemical Formula | C30H48O3 |
|---|
| Average Molecular Weight | 456.711 |
|---|
| Monoisotopic Molecular Weight | 456.360345406 |
|---|
| IUPAC Name | (1S,2R,4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid |
|---|
| Traditional Name | ursolic acid |
|---|
| CAS Registry Number | 77-52-1 |
|---|
| SMILES | [H][C@@]12[C@@H](C)[C@H](C)CC[C@@]1(CC[C@]1(C)C2=CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)[C@]3([H])CC[C@@]12C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 |
|---|
| InChI Key | WCGUUGGRBIKTOS-GPOJBZKASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-0ue9-2941000000-2ca89e826b7fdd2c8c1c | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0ue9-2941000000-2ca89e826b7fdd2c8c1c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-0023900000-43b07991e5cfcd6c9419 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0079-1000190000-a9eb6c04f4325a25f377 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-0a4i-0000930000-25139768b4507e334b4a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 30V, Negative | splash10-0a4i-0000900000-3ad5cc2bdb4d3808c5b8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 20V, Negative | splash10-0a4i-0000900000-63b4335e8110c54aba6e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 50V, Negative | splash10-0a4i-0000900000-b6287e2cceb1071deab7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-0a4i-0000900000-27e80ae2f29ac7c2825c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 40V, Negative | splash10-0a4i-0000900000-433a030e07383bf9ecbf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-0a4i-0000900000-315a19254ceeb5598e13 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-0a4i-0000900000-2eff827266b9664aa7cf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-0a4i-0000900000-ff12fe00efc56275c007 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-014l-0001223390-7f53534d3578e8cfc4b4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-0a4i-0000900000-3ad5cc2bdb4d3808c5b8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-0a4i-0000900000-63b4335e8110c54aba6e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-0a4i-0000900000-b6287e2cceb1071deab7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-0a4i-0000900000-27e80ae2f29ac7c2825c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-0a4i-0000900000-433a030e07383bf9ecbf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-01t9-0029800000-86a594c11680cf4a317f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-0udi-0001211390-507a9b205eaa7310f0ec | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-004i-0037900000-08b9c68893f80ab994c8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-0w9u-0001223691-a434f7aa9337860104e7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0001900000-147d46bee2af34cb0e9e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01vx-1005900000-1032db86d8a91ecc2551 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-055f-9468500000-8b9923090e53d44a56bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000900000-018366f20c7fedd5f9d2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fu-0003900000-ef66d040d6b775abb94e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-2008900000-0691b4da8771672d1def | View in MoNA |
|---|
|
|---|