| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:55:03 UTC |
|---|
| Update Date | 2020-04-22 15:10:55 UTC |
|---|
| BMDB ID | BMDB0002394 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
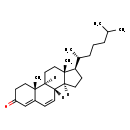
| Common Name | Cholesta-4,6-dien-3-one |
|---|
| Description | Cholesta-4,6-dien-3-one belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. Thus, cholesta-4,6-dien-3-one is considered to be a sterol. Based on a literature review a small amount of articles have been published on Cholesta-4,6-dien-3-one. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4,6-Cholestadien-3-one | HMDB, MeSH | | 4,6-Cholestadiene-3-one | HMDB | | Cholest-4,6-dien-3-one | MeSH, HMDB | | Cholesta-4,6-diene-3-one | MeSH, HMDB |
|
|---|
| Chemical Formula | C27H42O |
|---|
| Average Molecular Weight | 382.6218 |
|---|
| Monoisotopic Molecular Weight | 382.323565966 |
|---|
| IUPAC Name | (1S,2R,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-6,8-dien-5-one |
|---|
| Traditional Name | cholesta-4,6-dien-3-one |
|---|
| CAS Registry Number | 566-93-8 |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=CC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H42O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9-10,17-19,22-25H,6-8,11-16H2,1-5H3/t19-,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | XIWMRKFKSRYSIJ-GYKMGIIDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- Oxosteroid
- 3-oxosteroid
- Cyclohexenone
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gb9-2239000000-553c810e1d19e3831e2b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0bt9-0900000000-75bf7772949652267282 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0519000000-d3546c4987360df326ab | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-17d134ad7478d2eabf41 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0019000000-976c7ec9b4ecd0f35ce1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0api-3119000000-4c6c7aa38dc7223607b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-6239000000-e36074a482541cfecb97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-ab162bbf77e93fdedda9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0009000000-8854ae94fc792ba973a4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gba-2019000000-166d4a573bdfe5e55125 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-dcbeb6d0adb3f1962146 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066r-6594000000-093e658315d31d30c0c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-3921000000-1fa63eae5ed59c0d9041 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-334e990fc728bf944c79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0009000000-334e990fc728bf944c79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0049000000-c2d8d78e4aa615befe08 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Parish, Edward J.; Sun, Hang; Lu, Ding; Kizito, Stephen A.; Qiu, Zhihai. New chemical syntheses of cholest-4,6-dien-3-one. Lipids (2002), 37(12), 1197-1200. |
|---|