| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:54:59 UTC |
|---|
| Update Date | 2020-04-22 15:10:55 UTC |
|---|
| BMDB ID | BMDB0002391 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
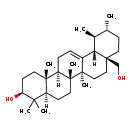
| Common Name | Uvaol |
|---|
| Description | Uvaol belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. Based on a literature review very few articles have been published on Uvaol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3.beta.)-urs-12-ene-3,28-diol | HMDB | | (3beta)-Urs-12-ene-3,28-diol | HMDB | | Urs-12-ene-3,28-diol | HMDB | | Urs-12-ene-3beta,28-diol | HMDB | | Urs-12-ene-3 beta,28-diol | HMDB | | Uvaol | MeSH |
|
|---|
| Chemical Formula | C30H50O2 |
|---|
| Average Molecular Weight | 442.728 |
|---|
| Monoisotopic Molecular Weight | 442.38108085 |
|---|
| IUPAC Name | (3S,4aR,6aR,6bS,8aS,11R,12S,12aS,14aR,14bR)-8a-(hydroxymethyl)-4,4,6a,6b,11,12,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-ol |
|---|
| Traditional Name | uvaol |
|---|
| CAS Registry Number | 545-46-0 |
|---|
| SMILES | [H]OC([H])([H])[C@]12C([H])([H])C([H])([H])[C@@]([H])(C([H])([H])[H])[C@]([H])(C([H])([H])[H])[C@@]1([H])C1=C([H])C([H])([H])[C@]3([H])[C@@]4(C([H])([H])[H])C([H])([H])C([H])([H])[C@]([H])(O[H])C(C([H])([H])[H])(C([H])([H])[H])[C@]4([H])C([H])([H])C([H])([H])[C@@]3(C([H])([H])[H])[C@]1(C([H])([H])[H])C([H])([H])C2([H])[H] |
|---|
| InChI Identifier | InChI=1S/C30H50O2/c1-19-10-15-30(18-31)17-16-28(6)21(25(30)20(19)2)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h8,19-20,22-25,31-32H,9-18H2,1-7H3/t19-,20+,22+,23-,24+,25+,27+,28-,29-,30-/m1/s1 |
|---|
| InChI Key | XUARCIYIVXVTAE-ZAPOICBTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03fr-0122900000-175477ab5c748536c9d7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-1021190000-48704668226df9f3244d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-0006-0100900000-4a0d73fcef1863afc64a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-0007-1920000000-d125a3fb0c728f5dbb40 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-059b-5900000000-fd6e8a3360a0b0a72052 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0000900000-1b790f5e9eb5bf2bf90e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-1231900000-29ce819c5908fe6bde31 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pvj-7694100000-2b9064a475a1e08a9d51 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000900000-7faf32cca5b2bea1eab2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-0001900000-ae3e9b87b7df659c3633 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-1009600000-56d6425409f73e689b7c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0001900000-28e870d4f02aa2fd80bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f9f-0693500000-7540a7ec9759094ded0b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-009l-1950100000-018a3d754cb4bbca193e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000900000-849799f7ece1fdb1b84b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0000900000-849799f7ece1fdb1b84b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05g3-0001900000-abd852c3c467163ae213 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|