| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:54:36 UTC |
|---|
| Update Date | 2020-04-22 15:10:48 UTC |
|---|
| BMDB ID | BMDB0002363 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Levuglandin E2 |
|---|
| Description | Levuglandin E2, also known as LGE2, belongs to the class of organic compounds known as long-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 13 and 21 carbon atoms. Thus, levuglandin E2 is considered to be an eicosanoid. Based on a literature review very few articles have been published on Levuglandin E2. |
|---|
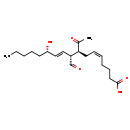
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 10,11-Seco-9,11-dioxo-15S-hydroxy-5Z,13E-prostadienoate | HMDB | | 10,11-Seco-9,11-dioxo-15S-hydroxy-5Z,13E-prostadienoic acid | HMDB | | LGE2 | HMDB |
|
|---|
| Chemical Formula | C20H32O5 |
|---|
| Average Molecular Weight | 352.4651 |
|---|
| Monoisotopic Molecular Weight | 352.224974134 |
|---|
| IUPAC Name | (5Z,8S,9R,10E,12S)-8-acetyl-9-formyl-12-hydroxyheptadeca-5,10-dienoic acid |
|---|
| Traditional Name | (5Z,8S,9R,10E,12S)-8-acetyl-9-formyl-12-hydroxyheptadeca-5,10-dienoic acid |
|---|
| CAS Registry Number | 91712-41-3 |
|---|
| SMILES | CCCCC[C@H](O)\C=C\[C@@H](C=O)[C@H](C\C=C/CCCC(O)=O)C(C)=O |
|---|
| InChI Identifier | InChI=1S/C20H32O5/c1-3-4-7-10-18(23)14-13-17(15-21)19(16(2)22)11-8-5-6-9-12-20(24)25/h5,8,13-15,17-19,23H,3-4,6-7,9-12H2,1-2H3,(H,24,25)/b8-5-,14-13+/t17-,18-,19+/m0/s1 |
|---|
| InChI Key | WJWAORNTZNRHBP-LBONXFAWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 13 and 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Long-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Long-chain fatty acid
- Branched fatty acid
- Hydroxy fatty acid
- Unsaturated fatty acid
- Ketone
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organooxygen compound
- Aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-9263000000-296c4cb7515a1b701ad6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-009x-9211700000-b71612e5c817a13ff0ec | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0019000000-126b7b23bc39fcdf3250 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-4589000000-1ae21424ce78470e10f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9351000000-c53c0dbb0d045d1c9b2c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-9ccba837b9402891d62b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fsi-1927000000-5696407cfccd87b39eee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aou-9510000000-72d76c85a734ac002076 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ap0-0129000000-7e6a9daa4876d6b8223f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0avj-9365000000-1c01e58986a6aa66a04e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05r0-9410000000-e23687ff0d093af89b31 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ue9-0019000000-b418a2943f101d719110 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uec-3459000000-fcf04b5aafdc13ca87cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abc-9641000000-6c6e011bd1ef9ed2b717 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Miller, Donald B.; Raychaudhuri, Swadesh R.; Avasthi, Kamlakar; Lal, Kasturi; Levison, Bruce; Salomon, Robert G. Prostaglandin endoperoxides. 25 Levuglandin E2: enantiocontrolled total synthesis of a biologically active rearrangement product from the prostaglandin endoperoxide PGH2. J. Org. Chem. 1990(55) 3164-3175 |
|---|