| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:54:25 UTC |
|---|
| Update Date | 2020-05-11 20:45:12 UTC |
|---|
| BMDB ID | BMDB0002349 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | trans-trans-Muconic acid |
|---|
| Description | trans-trans-Muconic acid, also known as (e,e)-muconic acid or (2E,4E)-2,4-hexadienedioate, belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. Based on a literature review a significant number of articles have been published on trans-trans-Muconic acid. |
|---|
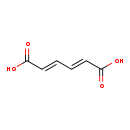
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,4E)-2,4-Hexadienedioic acid | ChEBI | | (e,e)-2,4-Hexadienedioic acid | ChEBI | | (e,e)-Muconic acid | ChEBI | | trans,trans-1,3-Butadiene-1,4-dicarboxylic acid | ChEBI | | trans,trans-2,4-Hexadienedioic acid | ChEBI | | trans,trans-Buta-1,3-diene-1,4-dicarboxylic acid | ChEBI | | (2E,4E)-2,4-Hexadienedioate | Generator | | (e,e)-2,4-Hexadienedioate | Generator | | (e,e)-Muconate | Generator | | trans,trans-1,3-Butadiene-1,4-dicarboxylate | Generator | | trans,trans-2,4-Hexadienedioate | Generator | | trans,trans-Buta-1,3-diene-1,4-dicarboxylate | Generator | | trans-trans-Muconate | Generator | | cis,cis-Muconate | MeSH | | Muconic acid | MeSH | | Muconic acid, (e,e)-isomer | MeSH | | trans-beta-Hydromuconic acid | MeSH | | 1,3-Butadiene-1,4-dicarboxylate | HMDB | | 1,3-Butadiene-1,4-dicarboxylic acid | HMDB | | Hexa-2,4-dienedioate | HMDB | | Hexa-2,4-dienedioic acid | HMDB | | trans,trans-Muconic acid | HMDB |
|
|---|
| Chemical Formula | C6H6O4 |
|---|
| Average Molecular Weight | 142.1094 |
|---|
| Monoisotopic Molecular Weight | 142.02660868 |
|---|
| IUPAC Name | (2E,4E)-hexa-2,4-dienedioic acid |
|---|
| Traditional Name | trans, trans-muconic acid |
|---|
| CAS Registry Number | 3588-17-8 |
|---|
| SMILES | OC(=O)\C=C\C=C\C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H6O4/c7-5(8)3-1-2-4-6(9)10/h1-4H,(H,7,8)(H,9,10)/b3-1+,4-2+ |
|---|
| InChI Key | TXXHDPDFNKHHGW-ZPUQHVIOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Medium-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain fatty acid
- Unsaturated fatty acid
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 301 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.2 mg/mL at 15 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-00di-3950000000-4833ed03fecc5d107087 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-00di-3950000000-4833ed03fecc5d107087 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0002-0920000000-25984cbefee561769fe3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-9400000000-1d771b996d6b60d97202 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00dj-8930000000-43cca1649a383c178957 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-1900000000-ba0597e9fe553580b776 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004m-9700000000-bb11fdbd4cb83319bdcf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9000000000-60049330475bcfa51fdd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1900000000-d089e6c88da6929f4c62 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-5900000000-d4f0aa2255b01aabc0bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fdp-9300000000-bc5a2d5e39f202b87d9c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-9600000000-3e5c79b293ac84a6a831 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9200000000-b7fce2b042b06e3cb276 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-9000000000-5b8045afa89b9f96b99d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9200000000-5b62b2b14bc9b75cd848 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fb9-9000000000-20df0bac53b1a654f39b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fb9-9000000000-e2a3f3d1e8945188e022 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|