| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:54:18 UTC |
|---|
| Update Date | 2020-04-22 15:10:43 UTC |
|---|
| BMDB ID | BMDB0002341 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 8-iso-15-keto-PGE2 |
|---|
| Description | 8-iso-15-keto-PGE2 belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid. Based on a literature review very few articles have been published on 8-iso-15-keto-PGE2. |
|---|
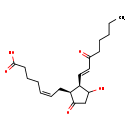
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5Z,8b,11a,13E)-11-Hydroxy-9,15-dioxo-prosta-5,13-dien-1-Oate | HMDB | | (5Z,8b,11a,13E)-11-Hydroxy-9,15-dioxo-prosta-5,13-dien-1-Oic acid | HMDB | | 8-Iso-15-keto-prostaglandine e2 | HMDB | | (5Z)-7-[(1S,2R)-3-Hydroxy-5-oxo-2-[(1E)-3-oxooct-1-en-1-yl]cyclopentyl]hept-5-enoate | HMDB |
|
|---|
| Chemical Formula | C20H30O5 |
|---|
| Average Molecular Weight | 350.4492 |
|---|
| Monoisotopic Molecular Weight | 350.20932407 |
|---|
| IUPAC Name | (5Z)-7-[(1S,2R)-3-hydroxy-5-oxo-2-[(1E)-3-oxooct-1-en-1-yl]cyclopentyl]hept-5-enoic acid |
|---|
| Traditional Name | 8-iso-15-keto-PGE2 |
|---|
| CAS Registry Number | 914804-63-0 |
|---|
| SMILES | CCCCCC(=O)\C=C\[C@H]1C(O)CC(=O)[C@H]1C\C=C/CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H30O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,16-17,19,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t16-,17+,19?/m0/s1 |
|---|
| InChI Key | YRTJDWROBKPZNV-LSAJIUQUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Prostaglandins and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Prostaglandin skeleton
- Long-chain fatty acid
- Hydroxy fatty acid
- Cyclopentanol
- Unsaturated fatty acid
- Fatty acid
- Alpha,beta-unsaturated ketone
- Cyclic alcohol
- Acryloyl-group
- Enone
- Secondary alcohol
- Cyclic ketone
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052u-4493000000-0d0c2247610da9ad51de | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-002b-7318900000-e3deca635536990d3ff8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0019000000-3ef3528ac5a012ba1cf7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001j-3079000000-e1ebbca36295283f32e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9452000000-aa26e5f3571b762113f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001j-0019000000-319ec8417f062af25ae2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-008i-0094000000-ecf8159ba7722550f80a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053u-9270000000-4f5b9ab71a4cd04afdc9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00lr-0029000000-9303b63bd2ebcbb5c384 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-067i-2195000000-4191e15aa7f06f807e38 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9210000000-7c06db5bb5cf1359caab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-0019000000-e747c20277d6c7786fa3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-6369000000-c74c0a7bb044927841f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9200000000-e6002eb037e36f06c242 | View in MoNA |
|---|
|
|---|