| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:53:51 UTC |
|---|
| Update Date | 2020-04-22 15:10:35 UTC |
|---|
| BMDB ID | BMDB0002304 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
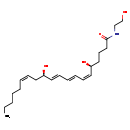
| Common Name | Leukotriene B4 ethanolamide |
|---|
| Description | Leukotriene B4 ethanolamide, also known as LTB4 ethanol amide, belongs to the class of organic compounds known as n-acylethanolamines. N-acylethanolamines are compounds containing an N-acyethanolamine moiety, which is characterized by an acyl group is linked to the nitrogen atom of ethanolamine. Thus, leukotriene B4 ethanolamide is considered to be an eicosanoid. Based on a literature review a small amount of articles have been published on Leukotriene B4 ethanolamide. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| LTB4 Ethanol amide | HMDB | | N-(2-Hydroxyethyl)-5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraen-1-amide | HMDB |
|
|---|
| Chemical Formula | C22H37NO4 |
|---|
| Average Molecular Weight | 379.5335 |
|---|
| Monoisotopic Molecular Weight | 379.272258677 |
|---|
| IUPAC Name | (5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxy-N-(2-hydroxyethyl)icosa-6,8,10,14-tetraenamide |
|---|
| Traditional Name | LTB4 ethanol amide |
|---|
| CAS Registry Number | 877459-63-7 |
|---|
| SMILES | CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(=O)NCCO |
|---|
| InChI Identifier | InChI=1S/C22H37NO4/c1-2-3-4-5-6-9-13-20(25)14-10-7-8-11-15-21(26)16-12-17-22(27)23-18-19-24/h6-11,14-15,20-21,24-26H,2-5,12-13,16-19H2,1H3,(H,23,27)/b8-7+,9-6-,14-10+,15-11-/t20-,21-/m1/s1 |
|---|
| InChI Key | DQLVVNIINUTUIU-XLFGVTECSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acylethanolamines. N-acylethanolamines are compounds containing an N-acyethanolamine moiety, which is characterized by an acyl group is linked to the nitrogen atom of ethanolamine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | N-acylethanolamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acylethanolamine
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08fr-4569000000-07bbcd45df0fab107131 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00al-9403580000-183af0e09936b9778485 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-2019000000-4f352551829a8f9e3932 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-9114000000-a9957a9113512b82fed1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-9211000000-bf01e60a47f738449f5c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-103935c4a5640462193d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-3119000000-77b68bbcfe920f285fb9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ox-9010000000-8161d2163618ea5160ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0019000000-680ed762e070f11a0bd6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-4249000000-001753066a308396c126 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cdi-9221000000-e87198640faaf65d8d77 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-12014e2c88deaf8a4a75 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-4339000000-639b80e888b3e42bb90c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-067l-9237000000-ed1d0e834f57d6d5ea83 | View in MoNA |
|---|
|
|---|