| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:52:19 UTC |
|---|
| Update Date | 2020-04-22 15:10:05 UTC |
|---|
| BMDB ID | BMDB0002189 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
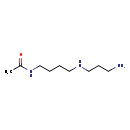
| Common Name | N8-Acetylspermidine |
|---|
| Description | N8-Acetylspermidine belongs to the class of organic compounds known as carboximidic acids. These are organic acids with the general formula RC(=N)-OH (R=H, organic group). N8-Acetylspermidine exists in all living species, ranging from bacteria to plants to humans. Based on a literature review a significant number of articles have been published on N8-Acetylspermidine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N(8)-Monoacetylspermidine | ChEBI | | N-[4-[(3-Aminopropyl)amino]butyl]-acetamide | HMDB | | N(8)-Acetylspermidine dihydrochloride | MeSH, HMDB | | N(8)-Acetylspermidine | MeSH, HMDB | | N-[4-[(3-Aminopropyl)amino]butyl]acetamide | HMDB | | N8-Acetylspermidine | HMDB | | N8-Monoacetylspermidine | HMDB |
|
|---|
| Chemical Formula | C9H21N3O |
|---|
| Average Molecular Weight | 187.2825 |
|---|
| Monoisotopic Molecular Weight | 187.168462309 |
|---|
| IUPAC Name | N-{4-[(3-aminopropyl)amino]butyl}acetamide |
|---|
| Traditional Name | N8-acetylspermidine |
|---|
| CAS Registry Number | 34450-15-2 |
|---|
| SMILES | CC(=O)NCCCCNCCCN |
|---|
| InChI Identifier | InChI=1S/C9H21N3O/c1-9(13)12-8-3-2-6-11-7-4-5-10/h11H,2-8,10H2,1H3,(H,12,13) |
|---|
| InChI Key | FONIWJIDLJEJTL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carboximidic acids. These are organic acids with the general formula RC(=N)-OH (R=H, organic group). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboximidic acids and derivatives |
|---|
| Sub Class | Carboximidic acids |
|---|
| Direct Parent | Carboximidic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Secondary aliphatic amine
- Carboximidic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 202 - 203 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001l-9200000000-7350029bbf7fca576698 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000i-0900000000-d04fb233b228bfc46b79 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-01w0-0900000000-e68fd1aa92642ae4208d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-03k9-7900000000-287b3decd26c7daeb830 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0089-9100000000-e42a1f8955afadd5f50d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0089-9000000000-b18801afe8921946e40f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-03k9-4900000000-fc04b14a414610552acd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1900000000-ed4e22e75d7e4ab076e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052o-5900000000-f486a2117b1dd060f58e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-dd7c2661d7b6091c7766 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-b573184691a2508c0c96 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-6900000000-9ecb44c4262e0df34ea4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-078ef81a1628477a273f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-009j-1900000000-ec737abcda867c6557b2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-6900000000-c57d66a60207b42d5604 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9100000000-5e4ff8c1235fb203f05f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-3aacf768b87a62171bf0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0229-9600000000-8855f267bbadb8cff170 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9100000000-286ce82869d4963e80f9 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Tabor, Herbert; Tabor, Celia W.; De Meis, Leopold. Chemical synthesis of N-acetyl-1,4-diaminobutane, N1-acetylspermidine, and N8-acetylspermidine. Methods Enzymol. (1971), 17(Pt. B), 829-33. |
|---|