| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:51:22 UTC |
|---|
| Update Date | 2020-04-22 15:09:47 UTC |
|---|
| BMDB ID | BMDB0002114 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
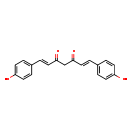
| Common Name | Bisdemethoxycurcumin |
|---|
| Description | Bisdemethoxycurcumin, also known as curcumin III, belongs to the class of organic compounds known as curcuminoids. These are aromatic compounds containing a curcumin moiety, which is composed of two aryl buten-2-one (feruloyl) chromophores joined by a methylene group. Based on a literature review a significant number of articles have been published on Bisdemethoxycurcumin. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,7-Bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione | ChEBI | | Bis(4-hydroxycinnamoyl)methane | ChEBI | | Bis(p-hydroxycinnamoyl)methane | ChEBI | | Curcumin III | ChEBI | | Didemethoxycurcumin | ChEBI | | 1,7-Bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione(e,e) | HMDB | | Bis-demethoxycurcumin | HMDB |
|
|---|
| Chemical Formula | C19H16O4 |
|---|
| Average Molecular Weight | 308.3279 |
|---|
| Monoisotopic Molecular Weight | 308.104859 |
|---|
| IUPAC Name | (1E,6E)-1,7-bis(4-hydroxyphenyl)hepta-1,6-diene-3,5-dione |
|---|
| Traditional Name | bisdemethoxycurcumin |
|---|
| CAS Registry Number | 24939-16-0 |
|---|
| SMILES | OC1=CC=C(\C=C\C(=O)CC(=O)\C=C\C2=CC=C(O)C=C2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C19H16O4/c20-16-7-1-14(2-8-16)5-11-18(22)13-19(23)12-6-15-3-9-17(21)10-4-15/h1-12,20-21H,13H2/b11-5+,12-6+ |
|---|
| InChI Key | PREBVFJICNPEKM-YDWXAUTNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as curcuminoids. These are aromatic compounds containing a curcumin moiety, which is composed of two aryl buten-2-one (feruloyl) chromophores joined by a methylene group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Diarylheptanoids |
|---|
| Sub Class | Linear diarylheptanoids |
|---|
| Direct Parent | Curcuminoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bis-desmethoxycurcumin
- Hydroxycinnamic acid or derivatives
- Styrene
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- 1,3-diketone
- Benzenoid
- 1,3-dicarbonyl compound
- Monocyclic benzene moiety
- Enone
- Acryloyl-group
- Alpha,beta-unsaturated ketone
- Ketone
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-0900000000-80dee0b7da47096385f3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01b9-4693400000-4dfcfa07fb4fcf08d15a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITTOF , negative | splash10-000f-0900000000-b6262ce6a5bc848338cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0319000000-51bdfbd78f352db8c233 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4j-0911000000-feb1ff110feb9ee4d644 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0690-3900000000-80607bb0dba3055c2ec4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0109000000-d6136d3ecd9a3055073b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0918000000-8da010127482f9dbe837 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07vl-1910000000-7b9ad6f38949b0ffed21 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ap0-0907000000-a0e824005455e435a22b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-0924000000-b8be7e1dc7f7aebc8446 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0920000000-3f12cad48a69d6a54824 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0309000000-59ab1e9ec81516f1adcb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-b4cdb8c134bf3a01b5cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0910000000-297489cfaed0405d2f7a | View in MoNA |
|---|
|
|---|