| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:48:05 UTC |
|---|
| Update Date | 2020-04-22 15:08:50 UTC |
|---|
| BMDB ID | BMDB0001844 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Methylsuccinic acid |
|---|
| Description | Methylsuccinic acid, also known as methylsuccinate or 2-methylbutanedioate, belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. Methylsuccinic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Methylsuccinic acid is a potentially toxic compound. |
|---|
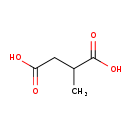
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methylsuccinate | Generator | | 2-Methylbutanedioic acid | HMDB | | Methylsuccinic acid, (+-)-isomer | HMDB | | (S)-2-Hydroxy-2-methylsuccinate | HMDB | | (S)-2-Hydroxy-2-methylsuccinic acid | HMDB | | (S)-Citramalate | HMDB | | (S)-Citramalic acid | HMDB | | 2-Methylbutanedioate | HMDB | | 2-Methylsuccinate | HMDB | | 2-Methylsuccinic acid | HMDB | | Methyl succinate | HMDB | | Methyl succinic acid | HMDB | | Methylbutanedioate | HMDB | | Methylbutanedioic acid | HMDB | | MEZ | HMDB | | Pyrotartarate | HMDB | | Pyrotartaric acid | HMDB |
|
|---|
| Chemical Formula | C5H8O4 |
|---|
| Average Molecular Weight | 132.1146 |
|---|
| Monoisotopic Molecular Weight | 132.042258744 |
|---|
| IUPAC Name | 2-methylbutanedioic acid |
|---|
| Traditional Name | methylsuccinic acid |
|---|
| CAS Registry Number | 498-21-5 |
|---|
| SMILES | CC(CC(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H8O4/c1-3(5(8)9)2-4(6)7/h3H,2H2,1H3,(H,6,7)(H,8,9) |
|---|
| InChI Key | WXUAQHNMJWJLTG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Methyl-branched fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methyl-branched fatty acid
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 117.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 516.5 mg/mL | Human Metabolome Project | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0002-0910000000-b99795b0e5069d573c2d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0002-0920000000-c96774045667ea1804df | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0002-0910000000-b99795b0e5069d573c2d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-007c-9100000000-bb48993db83be2104b10 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00du-9320000000-5f34c9f97642781028e4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-014i-3900000000-4afd6f9cd1309a7a392b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00kr-9600000000-e212a368f465b41cc1fa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0l0i-9200000000-c712b36382fde51f3ae5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-000i-9400000000-f709b1c905420a006038 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) 30V, Negative | splash10-0019-9400000000-40a1055d8102e323a077 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-000i-9400000000-f709b1c905420a006038 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0019-9400000000-40a1055d8102e323a077 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-5900000000-e50cb8068b9ea6aa88c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-9100000000-86a85f9d829697c248dd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-687b61805462d04b5ded | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-5900000000-4fd3ea5da8eb91f1951d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-9500000000-ec3019482baef20b293d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-23ddfbfc967e6df1168e | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0077-9000000000-1bd08b69cec7f9966cb2 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, DMSO-d6, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 22.53 MHz, DMSO-d6, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|