| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:44:09 UTC |
|---|
| Update Date | 2020-05-11 20:22:17 UTC |
|---|
| BMDB ID | BMDB0001324 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N'-Hydroxymethylnorcotinine |
|---|
| Description | N'-Hydroxymethylnorcotinine belongs to the class of organic compounds known as pyrrolidinylpyridines. Pyrrolidinylpyridines are compounds containing a pyrrolidinylpyridine ring system, which consists of a pyrrolidine ring linked to a pyridine ring. Based on a literature review very few articles have been published on N'-Hydroxymethylnorcotinine. |
|---|
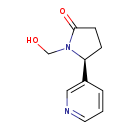
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5S)-1-(Hydroxymethyl)-5-(3-pyridinyl)-2-pyrrolidinone | HMDB | | (S)-1-(Hydroxymethyl)-5-(3-pyridinyl)-2-pyrrolidinone | HMDB | | N'-hydroxymethyl-norcotinine | HMDB | | N-(Hydroxymethyl)-norcotinine | HMDB | | N-Hydroxymethylnorcotinine | HMDB, MeSH | | 5-(3'-Pyridyl)-1-hydroxymethyl-2-pyrrolidone | MeSH, HMDB | | N-(Hydroxymethyl)norcotinine | MeSH, HMDB |
|
|---|
| Chemical Formula | C10H12N2O2 |
|---|
| Average Molecular Weight | 192.2145 |
|---|
| Monoisotopic Molecular Weight | 192.089877638 |
|---|
| IUPAC Name | (5S)-1-(hydroxymethyl)-5-(pyridin-3-yl)pyrrolidin-2-one |
|---|
| Traditional Name | (5S)-1-(hydroxymethyl)-5-(pyridin-3-yl)pyrrolidin-2-one |
|---|
| CAS Registry Number | 157129-55-0 |
|---|
| SMILES | OCN1[C@@H](CCC1=O)C1=CN=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C10H12N2O2/c13-7-12-9(3-4-10(12)14)8-2-1-5-11-6-8/h1-2,5-6,9,13H,3-4,7H2/t9-/m0/s1 |
|---|
| InChI Key | GQUFOBHEPVFQMD-VIFPVBQESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrrolidinylpyridines. Pyrrolidinylpyridines are compounds containing a pyrrolidinylpyridine ring system, which consists of a pyrrolidine ring linked to a pyridine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyrrolidinylpyridines |
|---|
| Direct Parent | Pyrrolidinylpyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrolidinylpyridine
- Alkaloid or derivatives
- Pyrrolidone
- 2-pyrrolidone
- N-alkylpyrrolidine
- Pyrrolidine
- Tertiary carboxylic acid amide
- Heteroaromatic compound
- Lactam
- Carboxamide group
- Alkanolamine
- Carboxylic acid derivative
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000x-8900000000-17e9e39a9b5afb58256d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fmu-9620000000-19d9b62ccf39d4748dee | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-2ce4c144463a7f9602f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-0900000000-bf4d3364aa1c4a6dd24d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0016-8900000000-0228fbf30573fb64ab04 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1900000000-d950c1f0ed34cdc857c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0900000000-c95a6479159dd1562f5b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-6900000000-ccd043d1f721b14556d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ox-1900000000-db05356c09c3425c9537 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fu-7900000000-a09c9d29c00ae37fa7d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9400000000-549ed23749cb2d367e67 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0900000000-c27938c67ab0c04df217 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-1900000000-ef03585c40e1d2f6cc14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9400000000-afd453d0634cceb28616 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|