| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:41:25 UTC |
|---|
| Update Date | 2020-04-22 15:06:46 UTC |
|---|
| BMDB ID | BMDB0001125 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
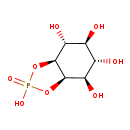
| Common Name | Inositol cyclic phosphate |
|---|
| Description | Inositol cyclic phosphate belongs to the class of organic compounds known as organic phosphoric acids and derivatives. These are organic compounds containing phosphoric acid or a derivative thereof. Inositol cyclic phosphate is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| D-Myo-inositol 1,2-cyclic phosphate | ChEBI | | Myo-inositol 1,2-cyclic phosphate | ChEBI | | 1D-Myo-inositol 1,2-cyclic phosphate | Kegg | | D-Myo-inositol 1,2-cyclic phosphoric acid | Generator | | Myo-inositol 1,2-cyclic phosphoric acid | Generator | | 1D-Myo-inositol 1,2-cyclic phosphoric acid | Generator | | Inositol cyclic phosphoric acid | Generator | | Inositol 1,2-cyclic phosphate | HMDB | | Inositol cyclic-1,2-monophosphate | HMDB | | Inositol cyclic phosphate, (D)-isomer | HMDB | | Myoinositol 1,2-cyclic phosphate | HMDB | | Inositol cyclic phosphate | MeSH |
|
|---|
| Chemical Formula | C6H11O8P |
|---|
| Average Molecular Weight | 242.1205 |
|---|
| Monoisotopic Molecular Weight | 242.01915384 |
|---|
| IUPAC Name | (3aR,4R,5S,6S,7R,7aS)-2,4,5,6,7-pentahydroxy-hexahydro-2H-1,3,2λ⁵-benzodioxaphosphol-2-one |
|---|
| Traditional Name | inositol 1,2-cyclic phosphate |
|---|
| CAS Registry Number | 43119-57-9 |
|---|
| SMILES | O[C@H]1[C@H]2OP(O)(=O)O[C@H]2[C@H](O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H11O8P/c7-1-2(8)4(10)6-5(3(1)9)13-15(11,12)14-6/h1-10H,(H,11,12)/t1-,2-,3+,4+,5-,6+/m0/s1 |
|---|
| InChI Key | SXHMVNXROAUURW-FTYOSCRSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organic phosphoric acids and derivatives. These are organic compounds containing phosphoric acid or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphoric acids and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Organic phosphoric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic phosphoric acid derivative
- Cyclitol or derivatives
- 1,3_dioxaphospholane
- Cyclic alcohol
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0089-2930000000-c749da37804623082943 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-016u-3329660000-e485841b1e6246c7e070 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-7eda9e0d437da3249fe2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002f-0190000000-ea57ccf34af155286eec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9200000000-1c996a9eb1c0a6fbcfed | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-90809cef8b443e05e700 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01vo-3980000000-3ba35ca42fc9cb1861ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06vi-9100000000-e0dc9b72c298686ab707 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-ff4f2501ddc7c779e19c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-c677579ba3bbddba0067 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fdn-9600000000-4cc6167012c54433b00d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-419db962ad2b749d1fb2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1090000000-73792d7f0d61cf2343ba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03fr-9300000000-61740087175d0110e32a | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|