| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:41:00 UTC |
|---|
| Update Date | 2020-04-22 15:06:40 UTC |
|---|
| BMDB ID | BMDB0001091 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Hydroxyquinine |
|---|
| Description | 3-Hydroxyquinine belongs to the class of organic compounds known as cinchona alkaloids. These are alkaloids structurally characterized by the presence of the cinchonan skeleton, which consists of a quinoline linked to an azabicyclo[2.2.2]octane moiety. Based on a literature review a significant number of articles have been published on 3-Hydroxyquinine. |
|---|
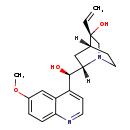
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Hydroxyquinidine | HMDB, MeSH | | 6'-Methoxycinchonan-3,9-diol | HMDB | | 3-Hydroxyquinidine, (3alpha,9S)-isomer | MeSH, HMDB | | 3-Hydroxyquinidine, (8alpha,9R)-isomer | MeSH, HMDB | | 3-Hydroxyquinine | MeSH |
|
|---|
| Chemical Formula | C20H24N2O3 |
|---|
| Average Molecular Weight | 340.4162 |
|---|
| Monoisotopic Molecular Weight | 340.178692644 |
|---|
| IUPAC Name | (3S,4R,6S)-3-ethenyl-6-[(R)-hydroxy(6-methoxyquinolin-4-yl)methyl]-1-azabicyclo[2.2.2]octan-3-ol |
|---|
| Traditional Name | 3-hydroxyquinidine |
|---|
| CAS Registry Number | 53467-23-5 |
|---|
| SMILES | [H][C@]12CCN(C[C@]1(O)C=C)[C@@]([H])(C2)[C@H](O)C1=CC=NC2=CC=C(OC)C=C12 |
|---|
| InChI Identifier | InChI=1S/C20H24N2O3/c1-3-20(24)12-22-9-7-13(20)10-18(22)19(23)15-6-8-21-17-5-4-14(25-2)11-16(15)17/h3-6,8,11,13,18-19,23-24H,1,7,9-10,12H2,2H3/t13-,18+,19-,20-/m1/s1 |
|---|
| InChI Key | BSRUJCFCZKMFMB-ZNYHDOEXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cinchona alkaloids. These are alkaloids structurally characterized by the presence of the cinchonan skeleton, which consists of a quinoline linked to an azabicyclo[2.2.2]octane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Cinchona alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Cinchona alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinchonan-skeleton
- 4-quinolinemethanol
- Quinoline
- Anisole
- Quinuclidine
- Alkyl aryl ether
- Aralkylamine
- Piperidine
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Tertiary alcohol
- 1,2-aminoalcohol
- Secondary alcohol
- Tertiary aliphatic amine
- Tertiary amine
- Organoheterocyclic compound
- Azacycle
- Ether
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic alcohol
- Alcohol
- Organic nitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-2901000000-46662034299736c6d91a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03k9-4192400000-35f213d793369e0c6fa1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-757d948be08bc83ac896 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-0409000000-0750a9802133c3ad7e0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-0911000000-f7b63c5080fbb178b639 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-c895e6c74087aa4b7c23 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dr-0119000000-8ff71092af9c3960da8e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uk9-0911000000-a23d26a2164f67414c08 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-6eb038badadf80b7dada | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000b-0489000000-9bb3e6ff8d6f7f01ae22 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fr-0901000000-5fd6aa84f8079b3fc2d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-3b43e0643b1819424eeb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0009000000-9364abb1565c929aab29 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00li-0961000000-7bb8ff583560fe8cdfb7 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Carroll, F. Ivy; Abraham, Philip; Gaetano, Kevan; Mascarella, S. Wayne; Wohl, Ronald A.; Lind, Joan; Petzoldt, Karl. (3S)-3-hydroxyquinidine, the major biotransformation product of quinidine. Synthesis and conformational studies. X-Ray molecular structure of (3S)-3-hydroxyquinidine methanesuiphonate. J. Chem. Soc., Perkin Trans. 1, 1991, 12, 3017-3026 |
|---|