Showing metabocard for Iodate (BMDB0001061)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-09-30 22:40:34 UTC | ||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2020-04-22 15:06:32 UTC | ||||||||||||||||||||||||||||||||||||||||||
| BMDB ID | BMDB0001061 | ||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||
| Metabolite Identification | |||||||||||||||||||||||||||||||||||||||||||
| Common Name | Iodate | ||||||||||||||||||||||||||||||||||||||||||
| Description | Iodate, also known as iodic acid or iodate (i2O62-), belongs to the class of inorganic compounds known as other non-metal halides. These are inorganic compounds containing 'other non-metals' and halogen. Based on a literature review a significant number of articles have been published on Iodate. | ||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||
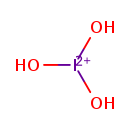
| Chemical Formula | H3IO3 | ||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 177.924 | ||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 177.91159 | ||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | trihydroxyiodanediium | ||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | trihydroxyiodanediium | ||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 15454-31-6 | ||||||||||||||||||||||||||||||||||||||||||
| SMILES | O[I++](O)O | ||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/H3IO3/c2-1(3)4/h2-4H/q+2 | ||||||||||||||||||||||||||||||||||||||||||
| InChI Key | VNFOOPCKMZASMJ-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of inorganic compounds known as other non-metal halides. These are inorganic compounds containing 'other non-metals' and halogen. | ||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||
| Class | Other non-metal halides | ||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Other non-metal halides | ||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Ontology | |||||||||||||||||||||||||||||||||||||||||||
| Status | Expected but not Quantified | ||||||||||||||||||||||||||||||||||||||||||
| Origin |
| ||||||||||||||||||||||||||||||||||||||||||
| Biofunction | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Application | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Cellular locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||
| Spectra | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Biospecimen Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Pathways |
| ||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0001061 | ||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB022400 | ||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 76615 | ||||||||||||||||||||||||||||||||||||||||||
| KEGG Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Iodate | ||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound | 84927 | ||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 29226 | ||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Scrabal, A.; Gruber, Josef. Halogenoxy compounds. XII. Kinetics of the formation of iodate from iodine in presence of triiodide ion. Monatshefte fuer Chemie (1917), 37 535-48. | ||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | ||||||||||||||||||||||||||||||||||||||||||