| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:39:57 UTC |
|---|
| Update Date | 2020-04-22 15:06:20 UTC |
|---|
| BMDB ID | BMDB0001016 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
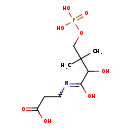
| Common Name | D-4'-Phosphopantothenate |
|---|
| Description | D-4'-Phosphopantothenate belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. D-4'-Phosphopantothenate is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). D-4'-Phosphopantothenate exists in all living species, ranging from bacteria to humans. D-4'-Phosphopantothenate participates in a number of enzymatic reactions, within cattle. In particular, D-4'-Phosphopantothenate can be biosynthesized from pantothenic acid; which is mediated by the enzyme pantothenate kinase 1. In addition, Cytidine triphosphate, D-D-d-4'-phosphopantothenate, and L-cysteine can be converted into cytidine monophosphate and 4'-phosphopantothenoylcysteine; which is mediated by the enzyme phosphopantothenate--cysteine ligase. In cattle, D-D-d-4'-phosphopantothenate is involved in the metabolic pathway called pantothenate and CoA biosynthesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| D-4'-Phosphopantothenic acid | Generator | | 4'-Phosphopantothenate | MeSH | | Phosphopantothenic acid | MeSH | | Phosphopantothenic acid, calcium salt (2:1) | MeSH | | Phosphopantothenic acid, calcium salt, (R)-isomer | MeSH | | (R)-4'-Phosphopantothenate | HMDB | | 4'-P-Pantothenate | HMDB |
|
|---|
| Chemical Formula | C9H18NO8P |
|---|
| Average Molecular Weight | 299.2149 |
|---|
| Monoisotopic Molecular Weight | 299.077003069 |
|---|
| IUPAC Name | 3-{2-hydroxy-3-methyl-3-[(phosphonooxy)methyl]butanamido}propanoic acid |
|---|
| Traditional Name | 3-{2-hydroxy-3-methyl-3-[(phosphonooxy)methyl]butanamido}propanoic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CC(C)(COP(O)(O)=O)C(O)C(O)=NCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H18NO8P/c1-9(2,5-18-19(15,16)17)7(13)8(14)10-4-3-6(11)12/h7,13H,3-5H2,1-2H3,(H,10,14)(H,11,12)(H2,15,16,17) |
|---|
| InChI Key | XHFVGHPGDLDEQO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Beta amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta amino acid or derivatives
- Monoalkyl phosphate
- Fatty amide
- Monosaccharide
- N-acyl-amine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Secondary alcohol
- Carboxamide group
- Secondary carboxylic acid amide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic nitrogen compound
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000t-9720000000-41704091b1bf13376017 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0002-9421200000-fd48be28cb334abd9b30 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-5392000000-359b42878651b9d9c2e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ul0-7960000000-5e1d3f9b32192536e41d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9200000000-f9de3989fd11e22afcbe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002b-9370000000-f7d945402a704f305bba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9200000000-14a239b738777adfeee4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-a3802c65fdf7fe039457 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|