| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:38:53 UTC |
|---|
| Update Date | 2020-04-22 15:06:02 UTC |
|---|
| BMDB ID | BMDB0000940 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Threonolactone |
|---|
| Description | Threonolactone belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. Based on a literature review a significant number of articles have been published on Threonolactone. |
|---|
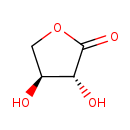
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| L-Threonic acid-1,4-lactone | ChEBI | | Threonic acid-1,4-lactone | ChEBI | | L-Threonate-1,4-lactone | Generator | | Threonate-1,4-lactone | Generator | | 3,4-Bis[(trimethylsilyl)oxy]dihydro-cis-2(3H)-furanone | HMDB | | Bis-TMS-threono-1,4-lactone | HMDB | | cis-Dihydro-3,4-bis[(trimethylsilyl)oxy]-2(3H)-furanone | HMDB | | Di-TMS-threono-1,4-lactone | HMDB |
|
|---|
| Chemical Formula | C4H6O4 |
|---|
| Average Molecular Weight | 118.088 |
|---|
| Monoisotopic Molecular Weight | 118.02660868 |
|---|
| IUPAC Name | (3R,4S)-3,4-dihydroxyoxolan-2-one |
|---|
| Traditional Name | (3R,4S)-3,4-dihydroxyoxolan-2-one |
|---|
| CAS Registry Number | 21730-93-8 |
|---|
| SMILES | O[C@H]1COC(=O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C4H6O4/c5-2-1-8-4(7)3(2)6/h2-3,5-6H,1H2/t2-,3+/m0/s1 |
|---|
| InChI Key | SGMJBNSHAZVGMC-STHAYSLISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Gamma butyrolactones |
|---|
| Direct Parent | Gamma butyrolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gamma butyrolactone
- Tetrahydrofuran
- Secondary alcohol
- Carboxylic acid ester
- 1,2-diol
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-63c53760143cd2044873 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fki-9430000000-f0cfa4e00daeb540aee7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-1900000000-d7d529471e146551a470 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-1900000000-7e70d7eac9b72e1cbd36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9100000000-7a95b8e64f0d366b4101 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-2900000000-c877a9861f0a9e0fa947 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-8900000000-874d13e11592fe389a74 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9000000000-295d5454144b0a74cb00 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9100000000-ccaaa47150cc978a00b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-9000000000-07ca8b081361da8d614e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-846eebab9a9caa26cce6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-6900000000-fde995765993269b5d05 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-9100000000-11a72527d4f24a67775b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9000000000-f1bef05a855750753132 | View in MoNA |
|---|
|
|---|