| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:38:20 UTC |
|---|
| Update Date | 2020-05-11 20:44:15 UTC |
|---|
| BMDB ID | BMDB0000901 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Valproic acid glucuronide |
|---|
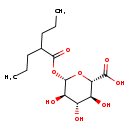
| Description | Valproic acid glucuronide, also known as 1-(2-propylpentanoate or dipropylacetate glucuronide, belongs to the class of organic compounds known as o-glucuronides. These are glucuronides in which the aglycone is linked to the carbohydrate unit through an O-glycosidic bond. Valproic acid glucuronide is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Valproate glucuronide | Generator | | 1-(2-Propylpentanoate | HMDB | | 1-(2-Propylpentanoate) beta-D-glucopyranuronate | HMDB | | 1-(2-Propylpentanoate) beta-D-glucopyranuronic acid | HMDB | | 1-(2-Propylpentanoate) beta-delta-glucopyranuronate | HMDB | | 1-(2-Propylpentanoate) beta-delta-glucopyranuronic acid | HMDB | | 1-(2-Propylpentanoic acid | HMDB | | 1-O-Valproyl-b-D-glucopyranuronic acid | HMDB | | 1-O-Valproyl-beta-delta-glucopyranuronic acid | HMDB | | 3,4,5-Trihydroxy-6-(2-propyl-pentanoyloxy)-tetrahydro-pyran-2-carboxylate | HMDB | | 3,4,5-Trihydroxy-6-(2-propyl-pentanoyloxy)-tetrahydro-pyran-2-carboxylic acid | HMDB | | Dipropylacetate glucuronide | HMDB | | Myproate | HMDB | | Myproic acid | HMDB | | Valproate | HMDB | | Valproic acid | HMDB | | VPA-g | HMDB | | VPAG | HMDB |
|
|---|
| Chemical Formula | C14H24O8 |
|---|
| Average Molecular Weight | 320.3356 |
|---|
| Monoisotopic Molecular Weight | 320.147117744 |
|---|
| IUPAC Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-[(2-propylpentanoyl)oxy]oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-[(2-propylpentanoyl)oxy]oxane-2-carboxylic acid |
|---|
| CAS Registry Number | 60113-83-9 |
|---|
| SMILES | CCCC(CCC)C(=O)O[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C14H24O8/c1-3-5-7(6-4-2)13(20)22-14-10(17)8(15)9(16)11(21-14)12(18)19/h7-11,14-17H,3-6H2,1-2H3,(H,18,19)/t8-,9-,10+,11-,14-/m0/s1 |
|---|
| InChI Key | XXKSYIHWRBBHIC-JVWRJRKNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-glucuronides. These are glucuronides in which the aglycone is linked to the carbohydrate unit through an O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glucuronides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-o-glucuronide
- O-glucuronide
- Beta-hydroxy acid
- Fatty acid ester
- Dicarboxylic acid or derivatives
- Hydroxy acid
- Monosaccharide
- Fatty acyl
- Oxane
- Pyran
- Carboxylic acid ester
- Secondary alcohol
- Oxacycle
- Acetal
- Carboxylic acid
- Polyol
- Carboxylic acid derivative
- Organoheterocyclic compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pds-9221000000-c4a088ea62b38f27162d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0006-7101290000-cfd964bab66e1a7f52c7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0902000000-2aede4223010fbfa28cd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-4900000000-8d4033ca0760506b6c4e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002e-9700000000-9b150785b1449fefe60e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00mo-2913000000-c1208d610d940b866f24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002f-4910000000-881718af4775dafc78f3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9700000000-2d69130ba045cf068e49 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1209000000-1fe9a50772ce5d04154f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4s-9301000000-7e6b8c6eaca54df62353 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-9100000000-b293f27db4ba83db2396 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0109000000-980c74ef8113693a80e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mp-3901000000-192aebcbdfefe9b795c9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052e-9400000000-1078233b81443bce6776 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Yamamura, Naotoshi; Muramatsu, Shigeki; Suzuki, Kanae; Uchiyama, Minoru; Nakajima, Eiichi. High-yield, enzymic synthesis of 14C- or 3H-labeled 1-O-valproyl-b-D-glucopyranuronic acid, the main metabolite of valproic acid in human. Radioisotopes (1999), 48( |
|---|