| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:37:47 UTC |
|---|
| Update Date | 2020-04-22 15:05:41 UTC |
|---|
| BMDB ID | BMDB0000869 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Uroporphyrin II |
|---|
| Description | Uroporphyrin II belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. Based on a literature review a significant number of articles have been published on Uroporphyrin II. |
|---|
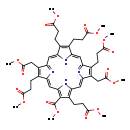
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Isouroporphyrin (II) octamethyl ester | HMDB | | Uroporphyrin I octamethyl ester | HMDB | | Methyl 9,19-bis(2-methoxy-2-oxoethyl)-5,10,14,15,20-pentakis(3-methoxy-3-oxopropyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaene-4-carboxylic acid | HMDB |
|
|---|
| Chemical Formula | C48H54N4O16 |
|---|
| Average Molecular Weight | 942.9596 |
|---|
| Monoisotopic Molecular Weight | 942.3534817 |
|---|
| IUPAC Name | methyl 9,19-bis(2-methoxy-2-oxoethyl)-5,10,14,15,20-pentakis(3-methoxy-3-oxopropyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaene-4-carboxylate |
|---|
| Traditional Name | uroporphyrin II |
|---|
| CAS Registry Number | 531-42-0 |
|---|
| SMILES | COC(=O)CCC1=C(CCC(=O)OC)/C2=C/C3=N/C(=C\C4=C(C(=O)OC)C(CCC(=O)OC)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(CCC(=O)OC)=C4CC(=O)OC)/C(CCC(=O)OC)=C3CC(=O)OC |
|---|
| InChI Identifier | InChI=1S/C48H54N4O16/c1-61-40(53)14-9-25-26(10-15-41(54)62-2)33-22-37-31(20-46(59)67-7)28(12-17-43(56)64-4)35(51-37)24-39-47(48(60)68-8)29(13-18-44(57)65-5)36(52-39)23-38-30(19-45(58)66-6)27(11-16-42(55)63-3)34(50-38)21-32(25)49-33/h21-24,49,52H,9-20H2,1-8H3/b32-21-,33-22-,34-21-,35-24-,36-23-,37-22-,38-23-,39-24- |
|---|
| InChI Key | JXFYRPWVBHUVFB-BGKHBZPASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-0000000096-a928397c25424ae241c6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05qi-0000000091-b4d6d121e7b31a5e9f36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-0000000090-517b06f58650cbfd00ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a7i-0000000098-35d787e1d1483933fc7b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0059-0000000092-4ddf070c250620e5ea59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01u0-0000000094-30d9925cac75c2221019 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000000090-b727d53615120b62028c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ug0-0000000090-77aadcd1d8871c2aa591 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zmi-0000000190-c88262c13df96cdf9ee2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000000293-0ffa1b0f6112c5f83736 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-0000000590-f1226455ce044e36d3cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01pt-1000000290-59b570ec2fcda1babcc0 | View in MoNA |
|---|
|
|---|