| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:34:16 UTC |
|---|
| Update Date | 2020-04-22 15:04:36 UTC |
|---|
| BMDB ID | BMDB0000649 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Clionasterol |
|---|
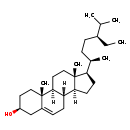
| Description | Clionasterol, also known as gamma-sitosterol or harzol, belongs to the class of organic compounds known as stigmastanes and derivatives. These are sterol lipids with a structure based on the stigmastane skeleton, which consists of a cholestane moiety bearing an ethyl group at the carbon atom C24. Thus, clionasterol is considered to be a sterol. Based on a literature review a significant number of articles have been published on Clionasterol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3beta,24S)-Stigmast-5-en-3-ol | ChEBI | | 22,23-Dihydroporiferasterol | ChEBI | | 24-Ethylcholest-5-en-3 beta-ol | ChEBI | | 24beta-Ethyl-5-cholesten-3beta-ol | ChEBI | | 24beta-Ethylcholesterol | ChEBI | | 24S-Ethylcholest-5-en-3beta-ol | ChEBI | | beta-Dihydrofucosterol | ChEBI | | gamma-Sitosterol | ChEBI | | Poriferast-5-en-3beta-ol | ChEBI | | (3b,24S)-Stigmast-5-en-3-ol | Generator | | (3Β,24S)-stigmast-5-en-3-ol | Generator | | 24-Ethylcholest-5-en-3 b-ol | Generator | | 24-Ethylcholest-5-en-3 β-ol | Generator | | 24b-Ethyl-5-cholesten-3b-ol | Generator | | 24Β-ethyl-5-cholesten-3β-ol | Generator | | 24b-Ethylcholesterol | Generator | | 24Β-ethylcholesterol | Generator | | 24S-Ethylcholest-5-en-3b-ol | Generator | | 24S-Ethylcholest-5-en-3β-ol | Generator | | b-Dihydrofucosterol | Generator | | Β-dihydrofucosterol | Generator | | g-Sitosterol | Generator | | Γ-sitosterol | Generator | | Poriferast-5-en-3b-ol | Generator | | Poriferast-5-en-3β-ol | Generator | | 24b-Ethylcholest-5-en-3b-ol | HMDB | | 24-Ethylcholesterol | HMDB | | 3beta-Stigmast-5-en-3-ol | HMDB | | Sitosterol | HMDB | | Sitosterol, (3beta,24xi)-isomer | HMDB | | Sitosterol, 26-(14)C-labeled | HMDB | | 3beta-Sitosterol | HMDB | | Harzol | HMDB | | beta-Sitosterol | HMDB | | Sitosterol, (3beta)-isomer | HMDB |
|
|---|
| Chemical Formula | C29H50O |
|---|
| Average Molecular Weight | 414.7067 |
|---|
| Monoisotopic Molecular Weight | 414.386166222 |
|---|
| IUPAC Name | (1S,2R,5S,10S,11S,14R,15R)-14-[(2R,5S)-5-ethyl-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol |
|---|
| Traditional Name | (1S,2R,5S,10S,11S,14R,15R)-14-[(2R,5S)-5-ethyl-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol |
|---|
| CAS Registry Number | 83-47-6 |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@H](C)CC[C@H](CC)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C29H50O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h10,19-21,23-27,30H,7-9,11-18H2,1-6H3/t20-,21+,23+,24+,25-,26+,27+,28+,29-/m1/s1 |
|---|
| InChI Key | KZJWDPNRJALLNS-FBZNIEFRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stigmastanes and derivatives. These are sterol lipids with a structure based on the stigmastane skeleton, which consists of a cholestane moiety bearing an ethyl group at the carbon atom C24. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Stigmastanes and derivatives |
|---|
| Direct Parent | Stigmastanes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - C24-propyl-sterol-skeleton
- Stigmastane-skeleton
- Triterpenoid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- Hydroxysteroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 147 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052v-1109000000-ae76f369c8c1b1b4fe0a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05fr-4103900000-a09c1c265657e57bd096 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0019500000-b9eddfa8d56747167ada | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-5139100000-42d184a108f9e994dc01 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9076000000-54ef98673160128cbacd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0002900000-158617c2e43f1a46b58a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0004900000-632250471791929b07e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-2019000000-717c6229722cf73f3faa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1118900000-620dc34d093f424c1d89 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-9163100000-4a80c636d0cb9659262f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9521000000-76b9e6176a167e0b61b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-eda302082c5114b2ab3a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0001900000-c37ac1577c1ddfa75134 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06r2-4016900000-e870c8bbee027c7c3a5a | View in MoNA |
|---|
|
|---|