| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:29:21 UTC |
|---|
| Update Date | 2020-05-11 20:20:32 UTC |
|---|
| BMDB ID | BMDB0000364 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3a,6a,7b-Trihydroxy-5b-cholanoic acid |
|---|
| Description | 3a,6a,7b-Trihydroxy-5b-cholanoic acid, also known as omega-muricholic acid or omega-mca, belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. Based on a literature review a significant number of articles have been published on 3a,6a,7b-Trihydroxy-5b-cholanoic acid. |
|---|
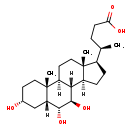
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3alpha,5beta,6alpha,7beta)-3,6,7-Trihydroxycholan-24-Oic acid | ChEBI | | Omega-mca | ChEBI | | Omega-muricholic acid | Kegg | | (3a,5b,6a,7b)-3,6,7-Trihydroxycholan-24-Oate | Generator | | (3a,5b,6a,7b)-3,6,7-Trihydroxycholan-24-Oic acid | Generator | | (3alpha,5beta,6alpha,7beta)-3,6,7-Trihydroxycholan-24-Oate | Generator | | (3Α,5β,6α,7β)-3,6,7-trihydroxycholan-24-Oate | Generator | | (3Α,5β,6α,7β)-3,6,7-trihydroxycholan-24-Oic acid | Generator | | Omega-muricholate | Generator | | 3a,6a,7b-Trihydroxy-5b-cholanoate | Generator | | 3a,6a,7b-Trihydroxy-5b-cholan-24-Oate | HMDB | | 3a,6a,7b-Trihydroxy-5b-cholan-24-Oic acid | HMDB | | W-Muricholate | HMDB | | W-Muricholic acid | HMDB | | Muricholic acid, (3alpha,5alpha,6alpha,7alpha)-isomer | HMDB | | Muricholic acid, (3alpha,5beta,6alpha,7alpha)-isomer | HMDB | | Trihydroxy-5 alpha-cholanoic acid | HMDB | | 3 alpha,6 alpha,7 beta-Trihydroxy-5 beta-cholanoic acid | HMDB | | alpha-Muricholic acid | HMDB | | Muricholic acid, (3alpha,5beta,6beta,7beta)-isomer | HMDB | | 3,6,7-Trihydroxy-5-cholanoic acid | HMDB | | Muricholic acid, sodium salt | HMDB | | beta-Muricholic acid | HMDB | | Hyocholic acid | HMDB | | Muricholic acid | HMDB | | Muricholic acid, (3alpha,5beta,6alpha,7beta)-isomer | HMDB | | Muricholic acid, (3alpha,5beta,6beta,7alpha)-isomer | HMDB |
|
|---|
| Chemical Formula | C24H40O5 |
|---|
| Average Molecular Weight | 408.5714 |
|---|
| Monoisotopic Molecular Weight | 408.28757439 |
|---|
| IUPAC Name | (4R)-4-[(1S,2R,5R,7R,8R,9R,10S,11S,14R,15R)-5,8,9-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid |
|---|
| Traditional Name | (4R)-4-[(1S,2R,5R,7R,8R,9R,10S,11S,14R,15R)-5,8,9-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid |
|---|
| CAS Registry Number | 6830-03-1 |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@H](C)CCC(O)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](O)[C@H](O)[C@]2([H])C[C@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C24H40O5/c1-13(4-7-19(26)27)15-5-6-16-20-17(9-11-23(15,16)2)24(3)10-8-14(25)12-18(24)21(28)22(20)29/h13-18,20-22,25,28-29H,4-12H2,1-3H3,(H,26,27)/t13-,14-,15-,16+,17+,18+,20+,21-,22-,23-,24-/m1/s1 |
|---|
| InChI Key | DKPMWHFRUGMUKF-NTPBNISXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Trihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Trihydroxy bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- 6-hydroxysteroid
- 7-hydroxysteroid
- 7-alpha-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Polyol
- Monocarboxylic acid or derivatives
- Organic oxide
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004l-0329000000-fed34af3d93baa3f7367 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-001i-1010029000-e5824ce563bff12a309c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0a4i-0001900000-d3a03d40e1e660b47856 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a4i-4984300000-003497742d7b2b6ee004 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-01p7-4920000000-7110ab8e5a5670619051 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-0009100000-aeaf5bcdde74fe1ddf76 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dm-0009000000-4af1ebd01d1d670d4f42 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003u-2209000000-454d741b691e592d251a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0007900000-e501198616f74730538f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-1009300000-9efc23876fa89e243bc6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9007000000-e5bd870e3f9870c89a14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0009600000-56beda528763ce5ae6df | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-059j-3449200000-98d256091462f2bbd882 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052u-4951000000-c203ad5efbf5e653aa09 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0001900000-68e3200734e1d0985f37 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-0009800000-a11781ffcf06d8bb288c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-3009300000-d354f18e540a94d7dfe1 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|